TRABAJOS ORIGINALES
Endoscopic and Endosonographic Management of Pancreatic Pseudocyst: a Long-Term Follow-Up
M. Dohmoto, K.Akiyama,Y.Iioka
RESUMEN
ANTECEDENTES:
En 72% de los casos, la causa de los quistes pancreáticos fue el
alcoholismo. El drenaje del quiste pancreático es sumamente crítico en
pacientes con várices estomacales. La ultrasonografía endoscópica (USE)
constituye un valioso complemento para el procedimiento de diagnóstico
con el fin de localizar el punto óptimo para realizar la punción y
evitar una hemorragia que podría producirse al dañar vasos sanguíneos,
ya sea intramurales o extramurales.
MÉTODOS: Se
realizó el drenaje por el método de drenaje pancreático retrógrado
endoscópico (DPRE), cistogastrostomía endoscópica (CGE) o
cistoduodenostomía endoscópica (CDE). Cuando existen dudas sobre la
existencia de várices y la identificación del quiste es difícil, el
drenaje transmural debe llevarse a cabo con control
endosonográfico.
RESULTADOS: Entre 1987 y
2002, se trataron 47 pacientes por seudoquistes pancreáticos,
utilizando el método de drenaje transmural o transductal - ADEUS. Se
realizó el drenaje guiado de un seudoquiste o absceso pancreático en 5
casos. En 42 pacientes, los seudoquistes pancreáticos desaparecieron
por completo. Seis pacientes presentaron una recurrencia entre 7 y 38
meses posteriores al retiro del drenaje. No se observó más recurrencias
en 22 pacientes durante el seguimiento de entre 5 y 11 años. En otros 6
pacientes se renovaron las prótesis debido a oclusión o descolocación.
Los 6 pacientes tuvieron que ser sometidos a cirugía, 3 de ellos debido
a recurrencia del quiste, 2 debido a drenaje insuficiente y 1 debido a
sangrado severo. No se registró ningún caso fatal relacionado con el
tratamiento endoscópico.
CONCLUSIÓN: La
ultrasonografía endoscópica constituye un valioso complemento en el
proceso de diagnóstico para localizar el punto óptimo para la punción y
evitar una hemorragia por daño de vasos sanguíneos intra o extramurales.
Las ventajas del drenaje endoscópico son invasividad mínima,
corto periodo de hospitalización y bajos costos. Estos aspectos hacen
que la terapia endoscópica sea el tratamiento mayormente elegido para
los seudoquistes pancreáticos.
Palabras claves: Seudoquiste pancreático, terapia endoscópica, ultrasonografía
SUMMARY
BACKGROUND:
The cause of pancreatic cyst were in 72 % due to alcoholism. A drainage
to pancreatic cyst is very critical for patient with the stomach
varices patient. The endoscopic ultrasonography(EUS) is a valuable
supplement to the diagnostic procedure to localise the optimal spot for
puncture and to avoid haemorrhage due to damage of intra or extra-mural
blood vessels.
METHODS: The drainage was reached
by transpapillary endoscopic retrograde pancreatic drainage (ERPD),
endoscopic cystogastrostomy (ECG) or endoscopic cystoduodenostomy (ECD).
The case that varices is doubted and If the identification of the cyst
is difficult the transmural drainage should be carried out under
endosonographic control.
RESULTS: Between
1987 and 2002, 47 patients had been treated for panceatic pseudocysts by
transmural or transductal drainage EUS-guided drainage of a pancreatic
pseudocyst or pancreatc abscess was carried out in 5 cases. In 42
patients pancreatic pseudocysts disappeared completely.
Six patients suffered a relapse 7 to 38 months after removal of the
drainage. No more recurrences were observed in 22 patients within
followed up 5-11 years. In another 6 patients the prostheses were
renewed because of occlusion or dislocation. Overall 6 patients had to
undergo surgery, 3 patients due to relapsing cyst, 2 patients because of
insufficient drainage and one patient because of severe bleeding. There
was no case of death related to the endoscopic treatment.
CONCLUSION:
The EUS is a valuable supplementation to the diagnostic procedure to
localize the optimal spot for puncture and to avoid haemorrhage because
of damage of intra- or extramural bloodvessels.
Advantages of
the endoscopic drainage are minimal invasiveness, short period of
hospitalization and low costs. These aspects make the endoscopic therapy
the first choice of treatment of pancreatic pseudocysts.
Key words: Pancreatic pseudocyst, endoscopic therapy, ultrasonographic.
INTRODUCTION
In pancreatic pseudocysts, endoscopic procedures represent a good
alternative to surgery such as cystogastrostomy or
cystojejunostomy. Many retrospective studies show that
endoscopic management is superior to surgical or
other interventional radiological techniques. The EUS is a valuable
supplementation to the diagnostic procedure to localise the optimal
spot for puncture and to avoid haemorrhage because of damage of intra-
or extra-mural bloodvessels. Advantages of the endoscopic drainage are
minimal invasiveness, short period of hospitalization and low costs.
PATIENTS AND METHODS
Between 1987 and 2002 , endoscopically and EUS-guided drainage of a
pancreatic pseudocyst or pancreatic abscess was carried out in 47
patients (29 men, 18 women: mean age 49, range (18-89). In 37 patients
(79 %) the pseudocysts were because of chronic pancreatitis. And the
main case of chronic pancreatitis was alcoholism in 34 cases (72 %). As
for the suspected patient of liver cirrhosis, endosonography was
undergone before the pseudocyst drainage. Retrogastric varices were
evident in one case. With 2 patients the pseudocysts were the result of a
traumatic or iatrogenic lesion of the pancreas, in 1 patient the cyst
was a result of a tumorous proliferation. In 5 patients etiology was
unknown.
40 patients (85 %) suffered from the
abdominal pain, 28 patients (60 %) reported loss of appetite and weight.
In 8 patients showed signs of a septicaemia and in 2 cases no symptoms
were present. In symptom-free patients there is an indication for
endoscopic drainage if the diameter of the cyst is larger than 5-6 cm
(1-4).
The diagnostic method prior to endoscopic
drainage includes the clinical examination, laboratory findings,
abdominal sonography, computed tomography (CT), magnetic resonance
imaging(MRI) and endoscopic retrograde cholangiopancreatography(ERCP).
Most of the pseudocysts were detected by means of sonography. The mean
size of the cysts was 8,9 cm (range 6-21 cm) (Fig.1).
The drainage was achivied by transpapillary endoscopic retrograde pancreatic drainage (ERPD)
(Fig.2), endoscopic cystogastrostomy (ECG)
(Fig.3)
or endoscopic cystoduodenostomy (ECD). ERPD and ECG are combined in
cases of multiple cysts if one cyst cannot be drained due to lack of
comunication with the pancreatic duct (Fig.4).
Generally the pseudocyst can be endoscopically localised as a prominent
impression of the gastric wall. If the endoscopic identification of the
cyst is difficult the transmural drainage should be performed under
endosonographic control.
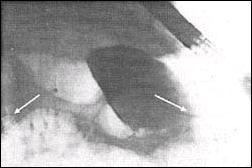
Fig. 1. CT scan showing the large pancreatic pseudocyst
(21 cm)
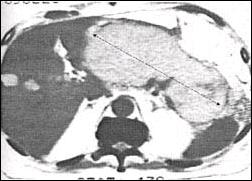
Fig. 2. Naso-cystic catheter is positioned transpapillary
into the pancreas pseudocyst
The aspiration of the cyst through the cystotom is done for
microbiological and cytological examinations. In case of purulent
infection the cyst is rinsed with isotonic saline. After retraction of
the cystotom a short pigtail prosthesis (7-10 French in diameter) or
nasocystic catheter are placed along the guidewire into the cyst (3).
Antacids and antibiotics are administered to all the patients.
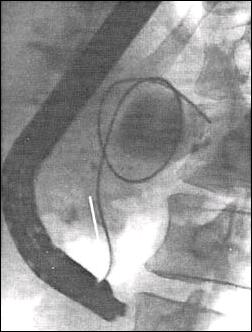
Fig. 3. Transgastrale pancreas pseudocyst drainage
with naso-cystic catheter

Fig. 4. Transgastrale and trans papillary combination drainage
with short prosthesis of the pancreas pseudocyst
RESULTS
Pseudocysts disappeared completely in 42 of 47 endoscopic drained patients (89%)
(Fig.5).
In the remaining 5 cases drainage was not successful. 6 (14 %) of
these 42 patients suffered a relapse until 38 months after removal of
the drainage. The patients whom went communication were 22 patients and
no other palindromia was not observed within a follow-up of 5 to 11
years . In 7 patients (15%) early complications such as occlusion or
dislocation of the prosthesis or bleeding into the cyst occured. In 6 of
these cases the prostheses were replaced because of malfunction. 6
patients had to undergo surgery, 3 patients because of relapsing cyst, 2
patients due to insufficient drainage of the cyst and one patient with a
bleeding which could not be treated endoscopically .
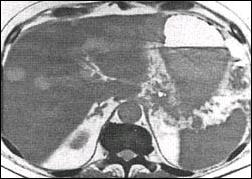
Fig. 5. The pancreatic pseudocyst disappeared completely
after the transgastrale drainage in 5 week
After disappearance of the abnormal clinical, sonographic and
endoscopic findings the prostheses were removed ambulatorily within 12
weeks (average 8.5 weeks) in case of ECG and ECD. After ERPD however the
drainage tubes were left in place 7 to 12 months because stable
recanalization of the stenosis and kinking of the main pancreatic duct
was required. Regularly the prostheses were renewed ambulatorily after a
period of 4 - 8 weeks to prevent occlusion. Any secondary infection of
cyst or other complication were not observed after endoscopic drainage. 3
patients died of laryngeal cancer, myocardial insufficency and diabetes
mellitus one, three and four years after drainage. There was no case of
death related to the endoscopic treatment. All patients (n=47)
described after the drainage a severe reduction in abdominal pain and/or
postprandial sensation of fullness. The average stay in hospital was 3
days (± 2 days).
DISCUSSION
Transmural puncture with the cystotom is easier to practise than the
dilation and drainage of a stenotic pancreatic duct. However if there is
a continous secretion of pancreatic juice through the prosthesis
because of obstruction of the duct secondarily, a transpapillary
dilation and drainage should be done. Large cysts with more than 5 cm in
diameter protruding the gastric wall can also be treated by transmural
drainage regardless of a connection with the pancreatic duct.
The endoscopic identification of pancreatic pseudocysts may be
difficult if there is no protrusion of the gastric or duodenal wall. In
these cases EUS-guided drainage of pseudocyst is precise and can be
performed even if the cyst dose not produce an endoscopic appear
bulge(5,6) (Fig. 6). The
advantages of this diagnostic procedure are: 1) exact localization of
the pseudocyst. 2) identification of retrogastral blood vessels (Fig.7).
3) differential diagnosis between malignant and benign cystic lesions
and intracystic haemorrhage. 4) precise punction with the cystotom under
EUS-control (Fig.8). 5) contrary to ERP, riskless use of EUS in case for acute pancreatitis. 6) measurement of the thickness of the cystic wall.
Fig. 6. EUS-document of corectly implanted guide and
nasocystc catheter in a pancreatic pseudocyt

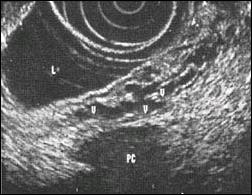
Fig. 7. Endoscopic ultrasonographic representation of
intra or extramural venectasia and search
for an avascular area to allow safe puncture.
V : varix, PC : Pancreas cyst, L : Lumen
The use of Doppler flow ultrasound allows diagnosis of important
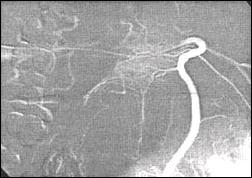
pseudocyst complications such as pseudoaneurysms and varices (7). But in
the non suspected patient of liver cirrhosis a big blood vessel was
showed by angiography in the extra wall of pancreatic cyst
(Fig.9).
In any case EUS is an excellent method to localise the optimal spot
for puncture of the cyst and to avoid haemorrhage due to injury of
intra- or extramural blood vessels. Proof of pseudocysts by EUS is
better than with CT(EUS 89% : CT 74%) especially with cysts of less than
2 cm diameter (EUS 83% : CT 33%) (8).
Technical
success rate of endoscopic drainage of pseudocysts are 71 - 100 %
(1-4,9-11). In our own study, transmural procedures of ECG and ECD were
successful in 100 % of cases. A complete disappearance of cysts after
endoscopic drainage is reported in 62 - 89 % of cases in the relevant
literature(2-4,9-12).
Fig. 8. EUS-guide puncture of a retrogastric pancreatric
pseudocyst with a cystotom using a Pentax FG-32 UA
echo endoscope, a Hitachi EUB-405 console
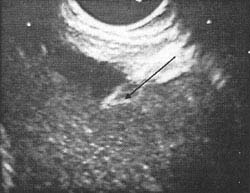
Fig. 9. Angiographic representation of a big blood vessel
of large pancreatic pseudocyst
There are several reasons for failure of endoscopic transmural
drainage: For example, if the wall of the pseuocyst measures over 10 mm,
the cyst is located in the pancreatic tail or the cyst is result from
acute necrotic pancreatitis (10). These cases are often primary
indications for laparoscopic or open access surgery.
Baron et al. reported successful treatment of necrotic pancreatitis by
endoscopic drainage and lavage with the help of a naso-cystic catheter
(13).
Nowadays the surgical management of
pseudocysts has changed to laparoscopic procedures like cystoenterostomy
(14-16). Oria et al. published positive results on laparoscopic
necrosectomy in cases of large acute pancreatic pseudocysts (14).
These operations however need general anaesthesia and the preoperative
work-up is more extensive than with endoscopic therapy. Therefore,
laparoscopic cystoenterostomy is a good alternative but it should be
performed only if there are contraindications for endoscopic drainage
or if endoscopy is unsuccessful. Laparoscopic operations took 123 min
(±15min) or 120 min (range 90 - 200min) and the postoperative stay in
hospital lasted 4 days (± 1day) to 7 days (range 5- 8days) (15,16).
These periods are much longer than for endoscopic access (45 ± 15
minutes, 3 ± 2 days).
Percutaneous drainage has a high
relapse tendency of 30 to 57 % and pancreaticocutaneous fistulas occur
in 40 % (17-19).
In the 1980s pseudocyst which
were older than 6 weeks were treated regularly by surgery or
percutaneous drainage with a catheter (20-22). Early pseudocysts with
thin walls too unstable for anastomosis can be treated by endoscopic
drainage at any time(3). Endoscopically placed pigtail prostheses
however may rapidly occlude in case of pancreatic necroses or septat
cysts, therfore naso-cystic tubes should be used for continous drainage
and lavage. Purulent cysts can also be treated by this way (1,13).
Endoscopic transmural drainage of an infected cyst is possible but only
after lavage with a lot of saline and under postprocedural antibiotic
cover. We did not find any secondary infection of cyst after endoscopic
drainage.
The incidence of pseudocyst within a chronic
pancreatitis is 20 - 40% (12,23). Between 20 and 60% of all pancreatic
pseudocysts spontaneously disappear within 6 weeks (24-27). No cysts
larger than 5 cm showed a spontaneous retrogression in our patients. In 8
- 20 % there are complications including pain, rupture of the cyst,
bleeding, fistulation or suppuration.
Beyond the 7
weeks of illness the rate of complications increase 20% each 5 weeks to
67% after 19 weeks (24-26). These complications are associated with
high rates of morbidity and lethality especially if emergency surgery is
required. Therefore, all pseudocysts larger than 5 cm whether
symptomatic or asymptomatic have to be treated. Soehendra et al.
recommends endoscopic drainage of all cysts remaining 2 - 3 months and
measuring 6 cm or greater (1).
According to
literature; complications like bleeding, infection, perforation,
exacerbation of pancreatitis, acute cholecystitis or duodenal
perforation come from 0-11 % after endoscopic drainage(2,4,9-12,28,29).
In contrast the rate of complication after cystojejunostomy is much
higher accounting for 14 - 41 % (23,30).
The rate of
recurrence after endoscopic drainage ranges between 6 and 23 % with a
long standing follow up (2,8). The mortality is 0 - 7 % (2-5,9-12).
In conclusion the EUS is a valuable supplementation to the diagnostic
procedure to localise the optimal spot for puncture and to avoid
haemorrhage because of damage of intra- or extramural bloodvessels.
Endoscopic internal drainage is a tolerable alternative to surgery
especially for high-risk patients.
Advantages of the
endoscopic drainage are minimal invasiveness, short period of
hospitalization and low costs. These aspects make the endoscopic therapy
the first choice of treatment of pancreatic pseudocysts.
REFERENCES
1. SOEHENDRA N, BINMOELLER KF, NAMVCH. Endoskopische Therapie am Pankreas. Endoskopie heute 1993;1:23-9
2. CREMER M, DEVIERE J, ENGELHOLM L. Endoscopic management of cyst and pseudo-cyst in chronic pancreatitis: long-term follow up after 7 years of experience. Gastrointest Endosc 1989;35:1-9
3. DOHMOTO M, RUPPKD, HÜNERBEIN M,SCHLAG PM. Endoskopische Drainage von Pankreaspseudozysten.Dtsch Med Wchschr 1995;120:1647-51
4. SAHEL J. Endoscopic drainage of pancreatic cysts. Endoscopy 1991;23:75-80
5. GRIMM H,BINMOELLER KF, SOEHENDRA N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc 1992;38:170-1
6. BINMOELLER KF, SOEHENDRA N. Endoscopic ultrasonography in the diagnosis and treatment of pancreatic pseudocysts. Gastrointest Endosc Clin N Am 1995;5:805-16
7. BRESLIN N,WALLACE MB. Diagnosis and fine needle aspiration of pancreatic pseudocysts: the role of endoscopic ultrasound.Gastrointest Endosc Clin N Am.2002 Oct;12(4):781-90
8. WIERSEMA MJ,KOCHMANN ML, HAWES RN. Endosonography compared with CT and ERCP in the evaluation of pancreatic pseudocysts. Gastrointes Endosc 1993;39:336-9
9. BINMOELLER KF, SEIFERT H, WALTER A, SOEHENDRA N. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 1995;42(3):219-24
10. BECKINGHAM IJ, KRIGE JE, BORMAN PC, TERBRANCHE J. Long term outcome of endoscopic drainage of pancreatic pseudocysts. Am J Gastroenterol 1999;94(1):71-4
11. SEIFERT H,DIETRICH C,SCHMITT T,CASPARY W,WEHRMANN T. Endoscopic ultrasound-guided one-step transmural drainage of cystic abdominal lesions with a large-channel echo endoscop. Endoscopy 2000;32(3):255-9
12. VITALE GC,LAWHON JC,LARSON GM,HARRELL DJ,REED DN JR,MACLEOD S. Endoscopic drainage pancreatic pseudocyst. Surgery 1999;126(4):616-21
13. BARON TH,THAGGAR DWG,MORGAN DE. Endoscopic Therapy for organized pancreatic necrosis. Gastroenterology 1996;111:755-64
14. ORIA A,OCAMPO C,ZANDALAZINI H,CHIAPETTA L,MORAN C. Internal drainage of giant acute pseudocysts:the role of video-assisted pancreatic necrosectomy. Arch Surg 2000;135(2):136-40
15. SMADJA C,BADAWY A,VONS C,GIRAUD V,FRANCO D. Laparoscopic cystogastrostomy for pancreatic pseudocyst is safe and effective. J Laparoendosc Adv Surg Tech 1999;9(5):401-3
16. CHAMPAULT G,RIZK N,LEBHAR E,CATHELINE JM,BARRAT C,CAZACU F. Laparoscopic treatment of pancreatic pseudocyst. 3 cases. Ann Chir 1998;52(1):41-4
17. HO CS,TAYLOR B. Percutaneous transgastric drainage for pancreatic pseudocyst. AJR 1984;143:623-5
18. ADAMS DB, ANDERSON BG. Percutaneous Catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg1992;215:571-6
19. BARTHET M,BUGALLO M,MOREIRA LS,BASTID C, SASTRE B,SAHEL J. Management of cyst and pseudocysts complikating chronic pancreatitis. Gastroentérol Clin Biol. 1993;17:270-6
20. BARKIN J,SMITH F, PEREIRAS R. Therapeutic percutaneous aspiration of pancreatic pseudocysts.Dig DisSci 1981;26:585-6
21. KARSON K,MARTIN E,FANKUCHEN E. Percutaneous drainage of pancreatic pseudocysts and abscesses. Radiology 1982;142:616-24
22. GERZOF S,JOHNSON W,ROBBINS A. Percutaneous drainage of infected pancreatic pseudocysts.Arch Surg 1984;119:888-93
23. DUNKIN BJ,PONSKY JL,HALE JC. Ultrasound-directed percutaneous cystgastrostomy for the treatment of a pancreatic pseudocyst. Surg Endosc 1998;12(12):1462-9
24. COLHOUN E, MURPHY JJ, MAC ERLEAN DP. Percutaneous drainage of pancreatic pseudocysts. Br J Surg. 1984;71:131-2
25. SONNNENBERG VAN,WITTICH E,CASOLA GR, Complicated pancreatic inflammatory dieseas: Diagnostic and therapeutic role of interventional Radiology.1985;155:335-40
26. BRADLEY E,CLEMENS J, GONZALES A. The natural history of pancreatic pseudocysts. A unified concept of management.Am J Surg 1979;137:135-41
27. REBER P,UHL W, BUCHLER M. Pancreatic pseudocysts in chronic pancreatitis. Differential diagnosis and therapy. Chirurg 1997;68(9):881-7
28. ZIPF A,POHLING KH,CASPARY WF, JUNG M. Endoskopische Therapie der chronischen Pankreatitis - Vorläufige Ergebnisse einer prospektiven Studie. Endoskopie heute.1994;4:289-94
29. WHITE SA,SUTTON CD,BERRY DP,CHILLSTONE D,REES Y,DENNISON AR, Experience of combined endoscopic percutaneous stenting with ultrasound guidance for drainage of pancreatic pseudocysts. Ann R Coll Surg Engl 2000;82(1): 11-5
30. RANSON JHC. The role of surgery in the management of acute pancreatitis. Ann Surg 1990;211:382-93